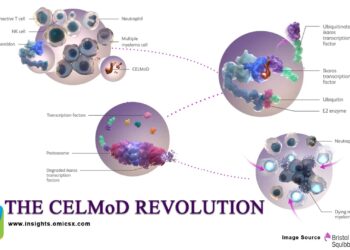
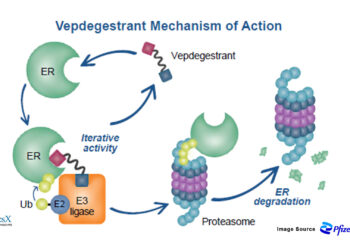
The CELMoD Revolution: How Bristol-Myers’ Iberdomide and Mezigdomide Could Redefine Multiple Myeloma Treatment with Billion-Dollar Market Impact
Bristol-Myers Squibb stands at the precipice of revolutionizing multiple myeloma treatment with two next-generation Cereblon E3 Ligase Modulators (CELMoDs) that...