What Is Vepdegestrant?
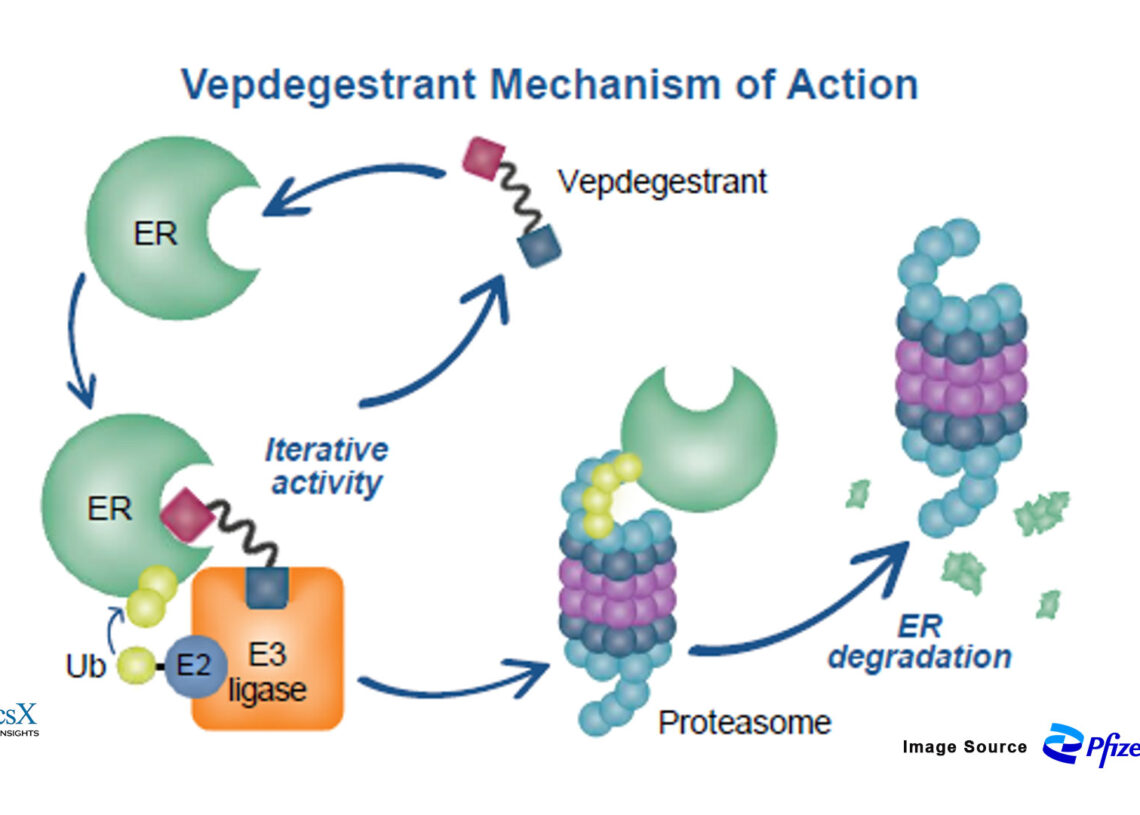
Vepdegestrant (ARV-471) is a PROTAC® (Proteolysis Targeting Chimera)-based oral SERD designed to degrade estrogen receptor alpha (ERα), a key driver in hormone receptor-positive breast cancers. Unlike traditional SERDs like fulvestrant, which block the receptor, vepdegestrant actively degrades it, potentially offering a more potent and sustained anti-tumor response. Vepdegestrant is jointly developed (50:50 ownership) by Arvinas and Pfizer.
Preclinical studies showed vepdegestrant could achieve up to 97% ER degradation, with the ability to overcome ESR1 mutations—an important feature in resistant tumors.
Vepdegestrant in Phase 3: How Strong Are Its Chances?
Based on recent clinical data and regulatory cues, vepdegestrant appears well-positioned for regulatory success in Phase 3, especially for patients harboring ESR1 mutations—a key population driving therapeutic resistance in ER+/HER2- breast cancer.
🔍 Strong Success Signals from VERITAC-2
🎯 Primary Endpoint Met in Target Population
The Phase 3 VERITAC-2 trial delivered robust results in the ESR1-mutant subgroup, which is the primary focus for regulatory approval:
-
43% reduction in risk of disease progression vs. fulvestrant
→ HR = 0.57 (95% CI: 0.42–0.77); P < 0.001 -
Median PFS improvement: 5.0 months vs. 2.1 months (fulvestrant)
-
Exceeded pre-specified regulatory HR threshold of 0.60
These results represent not just statistical significance, but clear clinical benefit in a group with significant unmet need.
🛣️ Regulatory Path Forward Looks Clear
Vepdegestrant has already earned an FDA Fast Track designation, suggesting strong regulatory confidence. Key advantages include:
-
Targeting ESR1-mutant population (~40%) in second-line settings
-
First PROTAC degrader to reach Phase 3 in oncology
-
Favorable safety profile, with discontinuation rates of 2.9% vs. 0.7% for fulvestrant
This creates a well-defined and de-risked path toward a potential New Drug Application (NDA) filing in H2 2025.
📊 Success Probability Assessment
✅ High Success Factors (Estimated 85–90%)
-
Statistically significant PFS improvement in ESR1m population
-
Meets FDA expectations for this defined subgroup
-
Novel mechanism as a PROTAC offering differentiated biology
-
Tolerable safety, enabling broader combination potential
-
Pfizer partnership ensures regulatory and commercial execution
⚠️ Residual Risks
-
Intent-to-treat population missed statistical significance
→ HR = 0.83; P = 0.07 -
Overall survival (OS) data remains immature (<25% events)
-
Crowded field of competing SERDs in late development stages
💼 Commercial Outlook: Targeted, But High Impact
While the likely label may focus initially on ESR1-mutant patients, this still represents a commercially meaningful subset:
🔹 Market Strengths
-
First-in-class PROTAC with clinically validated degradation
-
Addresses a resistance mechanism (ESR1 mutations) in up to 40% of post-CDK4/6 patients
-
ER+/HER2- breast cancer makes up ~70% of all breast cancers
-
Unmet need in patients relapsing after endocrine and CDK4/6 therapy
🤝 Strategic Backing
-
50/50 co-commercialization agreement with Pfizer
-
Leverages Pfizer’s global scale and oncology salesforce
📉 Analyst View
-
Evercore ISI: Results are “likely sufficient for approval in ESR1m patients”
-
Note: This narrows the market scope, but maintains a solid path to commercialization
🔚 Bottom Line: High Likelihood of Approval in 2026
Vepdegestrant is well-positioned for regulatory success in 2026, driven by:
-
Compelling Phase 3 efficacy in a clearly defined population
-
Regulatory momentum with Fast Track designation
-
A novel, first-in-class degradation mechanism
-
A manageable safety profile
Even if limited initially to ESR1-mutant patients, approval would validate the PROTAC platform broadly and further elevate the visibility of Targeted Protein Degradation (TPD) in oncology.