Bruton’s tyrosine kinase (BTK) has been a cornerstone target in B‑cell malignancies for over a decade, but resistance to BTK inhibitors has created a widening treatment gap for patients with relapsed or refractory disease. A new class of therapies—BTK degraders aims to close that gap by eliminating the BTK protein itself rather than merely inhibiting its activity, with BeiGene’s BGB‑16673 emerging as the front‑runner.
From Inhibition to Degradation: Why BTK Degraders Matter
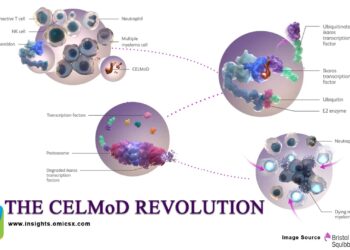
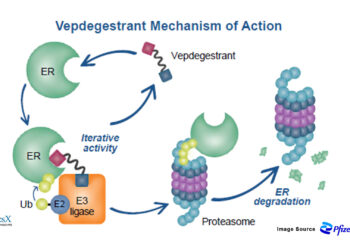
Traditional covalent and noncovalent BTK inhibitors block kinase activity but can be undermined by BTK mutations (such as C481S or L528W), altered signaling, and adaptive resistance. BGB‑16673, an oral “chimeric degradation activating compound” (cDAC), recruits an E3 ligase to tag BTK for ubiquitin‑mediated proteasomal destruction, suppressing both catalytic and scaffolding functions of BTK. Preclinical work shows BGB‑16673 degrades wild‑type and resistant BTK variants implicated in failure of both covalent and noncovalent inhibitors, and demonstrates central nervous system penetration—a potential advantage for difficult‑to‑treat compartments.
In first‑in‑human evaluations, BGB‑16673 reduced BTK protein levels in peripheral blood and tumor tissue, confirming on‑target engagement in patients.
Regulatory Tailwinds: Fast Track and A Global Phase III Program
The FDA granted Fast Track designation to BGB‑16673 for relapsed/refractory CLL/SLL, accelerating development and review for a therapy meeting a clear unmet need after BTK inhibitor progression. The program has rapidly advanced to Phase III across multiple geographies.
Two pivotal trials are now in motion:
- A Phase III head-to-head study comparing BGB‑16673 with investigator’s choice regimens (including idelalisib+rituximab) in relapsed/refractory CLL—designed to capture real‑world standards of care as comparators – NCT06970743
-
A randomized trial comparing BGB‑16673 against Eli Lilly’s Jaypirca (pirtobrutinib), a leading noncovalent BTK inhibitor, in relapsed/refractory CLL—an unambiguous test of degrader versus inhibitor in a post‑BTKi setting – NCT06973187
Global listings and trackers indicate Phase III initiation in 2025 across the United States and multiple international sites, reflecting broad momentum and investment behind the degrader strategy.
What Makes BGB‑16673 Different?
-
Degrades, not just inhibits: Eliminates BTK protein to shut down catalytic and scaffolding functions, potentially overcoming classic resistance pathways.
-
Active against resistant variants: Preclinical and early clinical data show activity against BTK mutations associated with covalent and noncovalent inhibitor resistance.
- Broad clinical activity: High response rates observed in heavily pretreated CLL/SLL, including those exposed to covalent BTKi, noncovalent BTKi, and BCL2 inhibitors, with encouraging 1‑year PFS.
-
Manageable safety and dosing flexibility: Early studies reported no maximum tolerated dose and responses starting at low doses, aiding dose optimization.
-
Regulatory and clinical leadership: Fast Track designation and multiple active Phase III studies position BGB‑16673 as the most advanced BTK degrader in the clinic.
-
FDA Fast Track Designation: Granted in August 2024 for relapsed/refractory CLL/SLL patients.
-
EMA PRIME Designation: Recently in 2025 granted for previously treated Waldenström macroglobulinemia.
-
Thus showing expansion beyond CLL: Signals in WM and other indolent lymphomas suggest potential label expansion if Phase II/III confirms cross‑indication benefit.
Market Size Projections
CLL/BTK Inhibitor Market Context
According to the latest market analysis, BeiGene is targeting what CEO John Oyler describes as the “$12 billion-plus CLL market”. This represents significant commercial opportunity given:
-
Market leadership potential: BeiGene’s Brukinsa has already surpassed AstraZeneca’s Calquence in quarterly sales ($828M vs $808M in Q4 2024)
-
Franchise building: BeiGene is working to build a “pre-eminent franchise” in the CLL space
BGB-16673 Specific Market Opportunity
Peak Sales Projections:
-
Phase II registrational data: Expected in 2026, which could support regulatory approval
-
Second-line positioning: Targeting patients who progress after BTK inhibitors, where limited options exist
-
Premium pricing potential: As the “most advanced BTK degrader in the clinic” with unique mechanism
Market Positioning Advantages:
-
First-mover advantage: Most advanced BTK degrader approaching regulatory approval
-
Resistance-overcoming capability: Designed to degrade both wildtype and mutant BTK forms
-
Unmet medical need: Addresses patients with limited options after BTK inhibitor progression
-
Strong clinical efficacy: 78% overall response rate in heavily pretreated patients
Commercial Timeline
Key Milestones:
-
2025: Phase III head-to-head trial initiation
-
2026: Potential Phase II registrational data readout
-
2026-2027: Regulatory submissions (BLA/MAA)
-
2027-2028: Expected approvals and market launch
-
2028-2030: Market penetration and revenue ramp
Competitive Market Context
BGB-16673 is positioned to compete directly in the BTK degrader space alongside Nurix’s NX-5948, with both showing similar efficacy profiles (76% vs 78% ORR respectively). However, BeiGene’s advantages include:
-
Earlier regulatory timeline: Better positioned for first-to-market advantage
-
Regulatory support: Both FDA Fast Track and EMA PRIME designations
-
Clinical advancement: Three concurrent Phase III trials vs competitors’ earlier-stage programs
-
Commercial infrastructure: Established hematology commercial capabilities through Brukinsa success
The combination of regulatory momentum, strong clinical data, and positioning in the large CLL market suggests BGB-16673 could generate significant commercial returns as a key component of BeiGene’s comprehensive BTK franchise strategy, potentially capturing substantial market share in the post-BTK inhibitor treatment setting
Redefining the BTK Pathway Playbook
If Phase III results validate Phase 1/2 performance, BTK degradation may become a new therapeutic backbone after BTK inhibitor failure and potentially move earlier in the treatment course for select patients. By addressing resistance at the protein level, BGB‑16673 could shift clinical algorithms away from cycling inhibitors toward a degradation‑first strategy in BTK‑driven malignancies.